Which Energy Changes Are Associated With A Liquid Freezing?
Liquid to Gas Stage Transition
Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase.
Learning Objectives
Describe the process of vaporization.
Key Takeaways
Central Points
- Evaporation is a phase transition from the liquid stage to the gas phase that occurs at temperatures below the boiling indicate at a given pressure level.
- For molecules of a liquid to evaporate, they must be located near the surface, be moving in the proper direction, and have sufficient kinetic free energy to overcome liquid-phase intermolecular forces.
- Boiling is a stage transition from the liquid phase to the gas stage that occurs at or in a higher place the boiling temperature.
- Boiling is the rapid vaporization of a liquid and occurs when a liquid is heated to its boiling point. A liquid's boiling indicate is the temperature at which the vapor pressure level of the liquid is equal to the pressure exerted on the liquid by the surrounding environment (air).
Fundamental Terms
- Vaporization: Vaporization is a phase transition from the liquid phase to the gas phase.
- manometer: An musical instrument to measure pressure in a fluid, peculiarly a double-legged liquid column gauge used to measure the deviation in the pressures of two fluids.
- Boiling: Boiling is the rapid vaporization of a liquid and occurs when a liquid is heated to its boiling point, or the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the surface of the liquid by the surrounding atmospheric gas (air).
- Evaporation: A blazon of vaporization of a liquid that only occurs on the liquid'southward surface.
Phase Transition: Liquid to Gas
Vaporization of a sample of liquid is a stage transition from the liquid phase to the gas phase. At that place are ii types of vaporization: evaporation and boiling.
- Evaporation occurs at temperatures below the boiling betoken, and occurs on the liquid'southward surface. For molecules of a liquid to evaporate, they must exist located near the surface, be moving in the proper management, and take sufficient kinetic energy to overcome intermolecular forces present in the liquid phase.
- Boiling, by contrast, is a rapid vaporization that occurs at or above the boiling temperature and at or below the liquid'south surface.
Vapor Pressure
In much the same fashion that tea spreads out from a tea bag once the bag is immersed in water, molecules that are bars within a phase will tend to spread themselves (and the thermal energy they acquit with them) equally widely as possible. This central law of nature is manifested in what is called the "escaping tendency" of the molecules from the phase. The escaping tendency is of fundamental importance in understanding all chemical equilibria and transformations.
It is possible to observe the trend of molecules to escape into the gas phase from a solid or liquid by placing the substance in a closed, evacuated container connected to a manometer for measuring gas pressure.
If this is washed with water, the fractional pressure of water Pw in the infinite above the liquid will initially be zero (step ane). Gradually, Pw will rise every bit molecules escape from the liquid phase and enter the vapor phase. At the same fourth dimension, some of the vapor molecules will condense back into the liquid phase (stride ii). Because this latter process is less favorable (at the particular temperature represented here), Pw continues to rise equally more than h2o vapor forms. Eventually a balance is reached betwixt the ii processes (step three), and Pw stabilizes at a fixed, or equilibrium, value Pvap, which depends on the substance and temperature. Pvap is known as the "equilibrium vapor force per unit area", or but every bit the "vapor pressure" of the liquid. The vapor pressure is a direct mensurate of the escaping tendency of molecules from a condensed land of matter.
Boiling
The boiling point is the temperature at which the vapor pressure level of the liquid is equal to the pressure exerted on the liquid by the surrounding environment (air). The escaping tendency of molecules from a stage always increases with temperature; therefore, the vapor pressure level of a liquid volition be greater at college temperatures. The variation of vapor pressure with temperature is not linear. Because the typical pressure at the earth's surface is 1 atm (760 torr), this is the vapor pressure that a liquid must equal in order for it to be at its normal humid point.

Vapor pressure and temperature: The variation of vapor pressure with temperature is not linear. The intercepts of each curve with the horizontal line at 1 atm (i.east. 760 torr) indicate the normal boiling indicate of each liquid, ranging from -25 °C for methyl chloride to over fourscore °C for fluorobenzene and 2-heptene.
The curve suggests that when the atmospheric pressure is lower than one atm (for instance, at higher altitudes), then the boiling point will occur at lower temperatures. This is because the liquid tin can exist heated less in order for its vapor force per unit area to equal the atmospheric pressure. This has indeed been observed to be true.
Related Phenomena Involving The Liquid Boundary Curvature
The vapor pressure level of a liquid is determined by the attractive forces that act on the molecules at the surface of a liquid. In a very small drop, the liquid surface is curved in such a way that each molecule experiences fewer nearest-neighbor attractions than is the instance for the bulk liquid. The outermost molecules of the liquid are bound to the droplet less tightly, and the drop has a larger vapor pressure than does the bulk liquid. If the vapor pressure level of the drop is greater than the partial pressure of vapor in the gas stage, the drop volition evaporate.
Interactive: Humid Point: Non-polar molecules (greyness) evaporate or boil more quickly than polar molecules (bluish and red). Attractions between molecules are shown with dotted lines. Run the model, then estrus the liquids. What does boiling look like at the molecular level?
A bubble is a pigsty in a liquid; molecules at the liquid boundary are curved inward, so they experience stronger nearest-neighbor attractions. As a outcome, the vapor pressure Pwestward of the liquid facing into a chimera is always less than that of the bulk liquid Pw at the aforementioned temperature. When the bulk liquid is at its normal boiling indicate (that is, when its vapor pressure is i atm), the pressure of the vapor within the bubble will exist less than i atm, and then the bubble will tend to collapse. Also, since the bubble is formed inside the liquid, the hydrostatic pressure level of the overlaying liquid will add together to this effect. For both of these reasons, a liquid volition not boil until the temperature is raised slightly above the humid point, a phenomenon known equally superheating. Once the humid begins, it volition continue to do so at the liquid's proper humid point.
Supercritical Fluids
A supercritical fluid is a substance at a temperature and pressure above its disquisitional point, where distinct liquid and gas phases do not be.
Learning Objectives
Discuss the backdrop of supercritical fluids.
Primal Takeaways
Central Points
- Supercritical fluids have backdrop between those of a gas and a liquid.
- A supercritical fluid can effuse through solids like a gas and dissolve materials like a liquid.
- All supercritical fluids are completely miscible with each other, and then for a mixture a single stage can be guaranteed, if the critical bespeak of the mixture is exceeded.
Key Terms
- supercritical fluid: Whatsoever substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist.
- critical temperature: The temperature across which no stage boundaries exist for a given substance.
- critical point: Also known every bit a critical state, this point occurs under weather (such as specific values of temperature, pressure level, or composition) at which no stage boundaries exist.
- critical force per unit area: The pressure beyond which no phase boundaries be for a given substance.
Properties of Supercritical Fluids
A supercritical fluid is whatever substance at a temperature and pressure in a higher place its critical bespeak, where singled-out liquid and gas phases do non be. This can exist rationalized by thinking that at loftier enough temperatures (above the critical temperature ) the kinetic energy of the molecules is high plenty to overcome any intermolecular forces that would condense the sample into the liquid phase. On the other hand, high enough pressures (above the disquisitional pressure ) would not allow a sample to stay in the pure gaseous state. Therefore, a balance between these two tendencies is achieved and the substance exists in a state betwixt a gas and a liquid.

Phase Diagram for a Substance: The figure highlights the critical point, higher up which (in either temperature or pressure level) the substance does not exist in either the liquid or gas stage. Under those conditions it is called a "supercritical fluid," and has properties betwixt those of a liquid and a gas.
It can effuse through solids (like a gas), and dissolve materials (like a liquid). In add-on, close to the disquisitional indicate, pocket-sized changes in pressure level or temperature result in big changes in density, allowing many properties of a supercritical fluid to exist "fine-tuned. " Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes. Carbon dioxide and water are the most commonly used supercritical fluids, every bit they are used for decaffeination and power generation, respectively.
In general terms, supercritical fluids have properties between those of a gas and a liquid. The critical properties of some substances used as solvents and equally supercritical fluids are shown in Table 1. Tabular array 2 shows density, diffusivity, and viscosity for typical liquids, gases, and supercritical fluids.
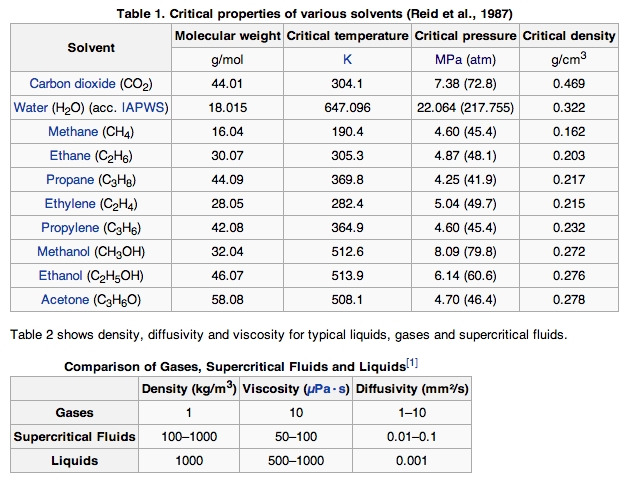
Disquisitional Properties of Various Solvents: Supercritical fluids have properties betwixt those of a gas and a liquid.
In addition, there is no surface tension in a supercritical fluid, every bit there is no liquid to gas phase purlieus. By irresolute the pressure and temperature of the fluid, the backdrop can exist "tuned" to exist more liquid- or gas-like. One of the nigh of import properties of supercritical fluids is their power to act as solvents. Solubility in a supercritical fluid tends to increment with the density of the fluid (at abiding temperature). Since density increases with pressure, solubility tends to increase with pressure.
The relationship with temperature is a little more complicated. At constant density, solubility will increase with temperature. Even so, close to the disquisitional point, the density tin drib sharply with a slight increase in temperature. Therefore, close to the critical temperature, solubility often drops with increasing temperature, then rises again.
All supercritical fluids are completely miscible with each other; therefore a single stage for a mixture can be guaranteed if the critical point is exceeded. The critical point of a binary mixture tin be estimated as the arithmetics hateful of the critical temperatures and pressures of the two components,
Tc(mix) = (mole fraction of A) x T c( A) + (mole fraction of B) x T c( B)
For greater accuracy, the disquisitional point can exist calculated using equations of state, such every bit the Peng Robinson or group contribution methods. Other backdrop, such every bit density, can as well be calculated using equations of country.
Case Report: Carbon Dioxide
In the pressure-temperature phase diagram of CO2, the humid separates the gas and liquid region and ends in the critical point, where the liquid and gas phases disappear to become a single supercritical phase. At well below the critical temperature, (eastward.g., 280 K), as the pressure level increases, the gas compresses and eventually (at just over 40 bar) condenses into a much denser liquid, resulting in the discontinuity in the line (vertical dotted line). The system consists of 2 phases in equilibrium, a dense liquid and a low density gas.

Stage Diagram for Carbon Dioxide: This diagram indicates the supercritical fluid region of CO2.
As the critical temperature is approached (300 M), the density of the gas at equilibrium becomes denser, and that of the liquid becomes lower. At the critical bespeak, (304.1 1000 and seven.38 MPa) there is no departure in density, and the ii phases become one fluid phase. Thus, above the critical temperature a gas cannot be liquified by pressure. At slightly above the critical temperature (310 K), in the vicinity of the disquisitional force per unit area, the line is almost vertical. A small increase in pressure causes a big increment in the density of the supercritical phase. Many other physical properties besides show big gradients with pressure near the critical point, such as viscosity, the relative permittivity, and the solvent forcefulness, which are all closely related to the density.
Liquid to Solid Phase Transition
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered to its freezing point.
Learning Objectives
Discuss the procedure of freezing.
Key Takeaways
Fundamental Points
- For nearly substances, the melting and freezing points are the same temperature; however, certain substances possess different solid – liquid transition temperatures.
- Most liquids freeze by crystallization, the formation of a crystalline solid from the uniform liquid.
- Freezing is nigh e'er an exothermic process, meaning that as liquid changes into solid, heat is released.
- The energy released upon freezing, known every bit the enthalpy of fusion, is a latent oestrus, and is exactly the same as the energy required to melt the same corporeality of the solid.
Key Terms
- Freezing: Freezing or solidification is a stage transition in which a liquid turns into a solid when its temperature is lowered to its freezing point.
- Nucleation: In the context of freezing, nucleation is the localized budding of a crystalline solid construction.
Freezing, or solidification, is a phase transition in which a liquid turns into a solid when its temperature is lowered to or below its freezing bespeak. All known liquids, except helium, freeze when the temperature is low enough. (Liquid helium remains a liquid at atmospheric pressure even at accented goose egg, and can be solidified just under college pressure level.)
For about substances, the melting and freezing points are the same temperature; still, certain substances possess different solid-liquid transition temperatures. For instance, agar displays a hysteresis in its melting and freezing temperatures: information technology melts at 85 °C (185 °F) and solidifies between 31 °C and 40 °C (89.6 °F to 104 °F).
Well-nigh liquids freeze by crystallization, the formation of a crystalline solid from the uniform liquid.

Crystalline Solid: Model of closely packed atoms within a crystalline solid.
Nucleation
This is a first-club thermodynamic stage transition, which means that as long as solid and liquid coexist, the equilibrium temperature of the system remains constant and equal to the melting signal. Crystallization consists of two major events: nucleation and crystal growth. Nucleation is the step in which the molecules kickoff to gather into clusters (on the scale of nanometers), arranging themselves in the periodic pattern that defines the crystal structure. The crystal growth is the subsequent growth of the nuclei that succeed in achieving and surpassing the critical cluster size.

Nucleation Leads to Crystal Formation: When saccharide is supersaturated in water, nucleation volition occur, allowing carbohydrate molecules to stick together and form large crystal structures.
Crystallization of pure liquids unremarkably begins at a lower temperature than the melting betoken, due to the high activation energy of homogeneous nucleation. The creation of a nucleus implies the formation of an interface at the boundaries of the new phase. Some energy is expended to form this interface, based on the surface free energy of each phase. If a hypothetical nucleus is besides small, the free energy that would exist released by forming its book is not enough to create its surface, and nucleation does not proceed. Freezing does not start until the temperature is low enough to provide enough energy to class stable nuclei.
In the presence of irregularities on the surface of the containing vessel, solid or gaseous impurities, pre-formed solid crystals, or other nucleators, heterogeneous nucleation may occur. Heterogeneous nucleation is when nucleation occurs on a surface that the substance is in contact with.
The melting bespeak of water at 1 atmosphere of pressure is very close to 0 °C (32 °F, 273.15 K), and in the presence of nucleating substances the freezing point of h2o is close to the melting bespeak. However, in the absence of nucleators water can supercool to -xl °C (-forty °F, 233 One thousand) before freezing. Under high pressure (two,000 atmospheres), water will supercool to as low as -70 °C (-94 °F, 203 One thousand) before freezing.
Freezing is Accompanied by Release of Heat
Freezing is almost always an exothermic process, meaning that as liquid changes into solid, heat is released. This may seem counterintuitive, since the temperature of the fabric does non rising during freezing (except if the liquid is supercooled). Simply rut must exist continually removed from the freezing liquid, or the freezing process will cease. The energy released upon freezing, known as the enthalpy of fusion, is a latent heat and is exactly the same every bit the free energy required to cook the same corporeality of the solid.
Interactive: Phase Change: Matter exists as solids, liquids and gases, and can modify state between these. The model shows a liquid material on the left (small atoms). The amount of estrus free energy is shown by kinetic energy (KE) shading, with deeper shades of red representing more energetic atoms. On the right side of the barrier is a solid material (large atoms). Run the model. How much free energy is able to penetrate the barrier? Remove the barrier. How rapidly exercise the more energetic atoms melt the solid?
Solid to Gas Stage Transition
Sublimation is the phase transition from the solid to the gaseous stage, without passing through an intermediate liquid stage.
Learning Objectives
Hash out the process of sublimation.
Primal Takeaways
Key Points
- Sublimation is an endothermic phase transition in which a solid evaporates to a gas.
- Solids that sublimate have such high vapor pressures that heating leads to a substantial vaporization even before the melting point is reached.
- The enthalpy of sublimation (also called heat of sublimation) can be calculated as the sum of the enthalpy of fusion and the enthalpy of vaporization.
Primal Terms
- sublimation: The procedure of transformation directly from the solid to the gaseous phase, without passing through an intermediate liquid phase.
- Triple point: In thermodynamics, the triple bespeak of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) coexist in thermodynamic equilibrium.
- degradation: A phase transition in which a gas is converted to solid, without passing though an intermediate liquid phase. It is the reverse process of sublimation.
Phase Transition: Solid to Gas
Sublimation is the procedure of transformation directly from the solid phase to the gaseous phase, without passing through an intermediate liquid phase. It is an endothermic phase transition that occurs at temperatures and pressures beneath a substance 's triple point (the temperature and pressure at which all three phases coexist) in its phase diagram.
At a given temperature, most chemical compounds and elements tin possess one of the three different states of thing at different pressures. In these cases, the transition from the solid to the gaseous land requires an intermediate liquid state. Only at temperatures below that of the triple point, a subtract in pressure level will event in a phase transition straight from the solid to the gaseous. As well, at pressures below the triple signal force per unit area, an increase in temperature will outcome in a solid existence converted to gas without passing through the liquid region.

Phase Diagram of a Pure Substance: Notice the triple point of the substance. At temperatures and pressures below those of the triple betoken, a stage modify between the solid and gas phases can take place.
For some substances, such as carbon and arsenic, sublimation is much easier than evaporation. This is because the pressure level of their triple point is very high and it is difficult to obtain them every bit liquids. The solid has such high vapor pressures that heating leads to a substantial amount of directly vaporization fifty-fifty earlier the melting betoken is reached.
The procedure of sublimation requires boosted energy and is therefore an endothermic change. The enthalpy of sublimation (also called estrus of sublimation) tin can be calculated equally the sum of the enthalpy of fusion and the enthalpy of vaporization.
The contrary process of sublimation is deposition (i.due east., gas to solid). For example, solid iodine, I2, is easily sublimed at temperatures around 100°C. Even ice has a measurable vapor pressure nigh its freezing betoken, as evidenced by the tendency of snow to evaporate in cold dry weather. There are other solids whose vapor pressure level overtakes that of the liquid before melting tin can occur. Such substances sublime; a common example is solid carbon dioxide (dry ice) at one atm of atmospheric pressure.

Dry out Ice: Solid carbon dioxide (known every bit "dry ice") sublimes into the air.
Heating Curve for Water
Water transitions from water ice to liquid to h2o vapor as heat is added to it.
Learning Objectives
Discuss the heating curve for water.
Key Takeaways
Key Points
- A heating curve graphically represents the phase transitions that a substance undergoes as heat is added to it.
- The plateaus on the curve mark the stage changes. The temperature remains constant during these phase transitions.
- Water has a high boiling point because of the strong hydrogen bonds between the water molecules; information technology is both a strong hydrogen bail donor and acceptor.
- The first change of phase is melting, during which the temperature stays the same while water melts. The 2d alter of phase is boiling, equally the temperature stays the same during the transition to gas.
Key Terms
- hydrogen bond: A strong intermolecular bond in which a hydrogen atom in one molecule is attracted to a highly electronegative atom (usually nitrogen or oxygen) in a different molecule.
- specific heat capacity: The amount of oestrus needed to raise the temperature of 1 g of a substance by 1 caste Celsius.
Like many substances, h2o can exist in dissimilar phases of matter: liquid, solid, and gas. A heating curve shows how the temperature changes as a substance is heated up at a constant charge per unit.
Drawing a Heating Curve
Temperature is plotted on the y-axis, while the x-axis represents the heat that has been added. A abiding charge per unit of heating is causeless, so that i can also think of the x-axis as the corporeality of time that goes past equally a substance is heated. There are two main observations on the measured curve:
- regions where the temperature increases as heat is added
- plateaus where the temperature stays constant.
It is at those plateaus that a phase alter occurs.
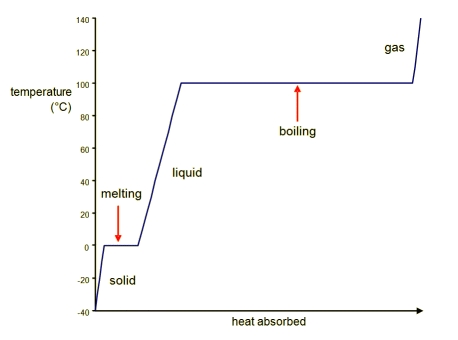
Heating Curve of Water: The stage transitions of h2o.
Assay of a Heating Bend
Looking from left to right on the graph, there are 5 distinct parts to the heating curve:
- Solid ice is heated and the temperature increases until the normal freezing/melting signal of naught degrees Celsius is reached. The amount of heat added, q, can be computed by: [latex]\text{q}=\text{m}\cdot \text{C}_{\text{H}_2\text{O}(\text{southward})}\cdot \Delta \text{T}[/latex], where m is the mass of the sample of water, C is the specific estrus capacity of solid water, or ice, and [latex]\Delta \text{T}[/latex] is the alter in temperature during the process.
- The start phase change is melting; as a substance melts, the temperature stays the aforementioned. For h2o, this occurs at 0o C. The in a higher place equation (described in part i of the bend) cannot be used for this part of the curve because the change in temperature is zero! Instead, use the estrus of fusion ([latex]\Delta \text{H}_{\text{fusion}}[/latex] ) to summate how much estrus was involved in that process: [latex]\text{q}=\text{m}\cdot \Delta \text{H}_{\text{fusion}}[/latex], where m is the mass of the sample of water.
- After all of the solid substance has melted into liquid, the temperature of the liquid begins to increase every bit heat is absorbed. It is then possible to calculate the heat captivated by: [latex]\text{q}=\text{m}\cdot \text{C}_{\text{H}_2\text{O}(\text{l})}\cdot \Delta \text{T}[/latex]. Notation that the specific heat capacity of liquid water is different than that of ice.
- The liquid will begin to boil when enough heat has been absorbed by the solution that the temperature reaches the boiling point, where again, the temperature remains abiding until all of the liquid has become gaseous water. At the atmospheric pressure of 1 atm, this phase transition occurs at 100o C (the normal boiling point of water). Liquid water becomes h2o vapor or steam when information technology enters the gaseous phase. Utilise the heat of vaporization ([latex]\Delta \text{H}_{\text{vap}}[/latex] ) to summate how much heat was absorbed in this procedure: [latex]\text{q}=\text{m}\cdot \text{C}_{\text{H}_2\text{O}(\text{k})}\cdot \Delta \text{T}[/latex], where m is the mass of the sample of water.
- After all of the liquid has been converted to gas, the temperature will proceed to increase every bit heat every bit added. Once again, the heat added that results in a certain modify temperature is given past: [latex]\text{q}=\text{m}\cdot \text{C}_{\text{H}_2\text{O}(\text{m})}\cdot \Delta T[/latex]. Notation that the specific heat capacity of gaseous water is dissimilar than that of water ice or liquid water.
- Water has a loftier boiling point considering of the presence of all-encompassing hydrogen bonding interactions between the h2o molecules in the liquid phase (water is both a strong hydrogen bond donor and acceptor). When heat is first applied to water, it must break the intermolecular hydrogen bonds inside the sample. After breaking the bonds, estrus is then captivated and converted to increased kinetic free energy of the molecules in order to vaporize them.
Source: https://courses.lumenlearning.com/boundless-chemistry/chapter/phase-changes/
Posted by: kingtordese.blogspot.com

0 Response to "Which Energy Changes Are Associated With A Liquid Freezing?"
Post a Comment